electronic configuration for iodine|Iodine (I) : Tuguegarao Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular . Tingnan ang higit pa Cinema movie schedule in Robinsons Place Pangasinan. Movies TV Food & Drink Shops & Services. Tickets Menu. Get Tickets. Ayala Malls Cinemas . Now Showing. Real Life Fiction. Opens Sep 4. Beetlejuice Beetlejuice. Manila. 26°C. Partly cloudy. Tue. 27°C. Wed. 28°C. Thu. 27°C. Powered by WeatherAPI.com. Forex.
PH0 · Iodine, electron configuration
PH1 · Iodine Electronic Configuration and Distribution in Shells
PH2 · Iodine Electron Configuration: Everything You Need to Know
PH3 · Iodine Electron Configuration: Everything You Need to Know
PH4 · Iodine Electron Configuration (I) with Orbital Diagram
PH5 · Iodine (I)
PH6 · Electron configuration of iodine 【Electron Configuration】 2022
PH7 · Electron configuration for Iodine (element 53). Orbital diagram
PH8 · Electron Configuration Chart of All Elements (Full Chart)
PH9 · Complete Electron Configuration for Iodine (I, I– ion)
PH10 · A step
PRC ID/License Renewal via Shipping Service Delivery//payment via GCASHHi everyone, this video is a step-by-step tutorial on the online renewal of our PRC ID.
electronic configuration for iodine*******Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of iodine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. In the iodine ground-state electron configuration, the last electrons of the 5p orbital are located in the . Tingnan ang higit paThe total number of electrons in iodine is fifty-three. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in iodine in . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit paAfter arranging the electrons, it is seen that the last shell of the iodine atom has seven electrons. Therefore, the valence electronsof iodine are seven. The elements . Tingnan ang higit paIodine (I) After arranging the electrons, it is seen that the last shell of the iodine atom has seven electrons. Therefore, the valence electronsof iodine are seven. The elements . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular . Tingnan ang higit pa
Mar 23, 2023
Electronic configuration of the Iodine atom. Valence electrons. Orbital diagram.
Iodine Electron Configuration: Iodine is a chemical element that has a symbol I. The atomic number of Iodine is 53. It is the heaviest of the stable halogens. It exists as a purple-black, lustrous, non-metallic .Electron configuration for iodine. The history of Iodine. Periodic table history. Identifiers. List of unique identifiers for Iodine in various chemical registry databases. Iodine is a .
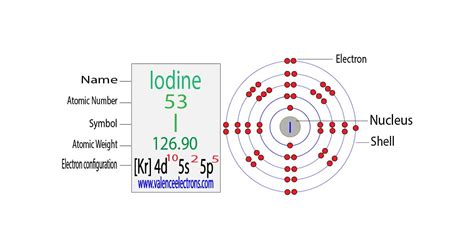
This article will discuss information on the electronic configuration of iodine, such as the writing of its electronic configuration and the orbital diagram of ground .Iodine. Full electron configuration of iodine: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. tellurium ← iodine → xenon.
A step-by-step description of how to write the electron configuration for Iodine (I). In order to write the I electron configuration we first need to know t.
Electronic Configuration of Iodine. The atomic number of Iodine (I) is 53. Therefore, the electronic configuration of Iodine can be represented as: 1s2 2s2 2p6 .
The Electron configuration of iodine is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s²4d¹⁰ 5p⁵. Iodine, also called iodine is defined as the chemical element that belongs to the periodic table. .
Similarly, the observed electron configuration of copper is [Ar]4s 1 3d 10 instead of [Ar]s 2 3d 9. The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns . The chemical symbol of Iodine is I. The atom of Iodine has an atomic number of 53. The electronic configuration of Iodine is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁵. Iodine has seven valence electrons. The most common valency of Iodine is .I (Iodine) is an element with position number 53 in the periodic table. Located in the V period. Melting point: 113.5 ℃. Density: 4.94 g/cm 3 . Electronic configuration of the Iodine atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 5Electronic configuration of the Iodine atom in ascending .The Electron configuration of iodine is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s²4d¹⁰ 5p⁵. Iodine, also called iodine is defined as the chemical element that belongs to the periodic table. It is located in group 17, more precisely in the halogens, its atomic number is 53 and it is represented by the symbol I. This element can be .
The atomic number of iodine is 53, which means it has 53 electrons. Now it is possible to find the orbital notation of iodine very easily through electron configuration. That is, the orbital notation of iodine is 1s 2 2s 2 2p . The electron configuration in the outer shell is \(ns^2np^5\). As the atomic number increases, the reactivity of the halogens decreases. Fluorine and chlorine exist as gases at room temperature, while bromine is a liquid, and iodine is a solid. Review. Pick two elements that are halogens. For each, write the name, chemical symbol, and atomic .electronic configuration for iodineThe radioactive isotope iodine-131 is sometimes used to treat cancerous thyroid glands. Iodine is an essential element for humans, who need a daily intake of about 0.1 milligrams of iodide. Our bodies contain up to 20 milligrams, mainly in the thyroid gland. This gland helps to regulate growth and body temperature.The electronic configuration of Iodine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. What is the abbreviated electronic configuration of Iodine? The abbreviated electronic configuration of Iodine is [Kr] 4d10 5s2 5p5. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas .
In this video we will write the electron configuration for I- the Iodide ion. We’ll also look at why Iodine forms a 1- ion and how the electron configuration.The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a .
The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Iodine is [Kr] 4d10 5s2 5p5.
Electron Configuration: 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 5s 2 p 5; Electrons per Energy Level: 2,8,18,18,7 Shell Model; . Iodine - I (EnvironmentalChemistry.com)- Comprehensive information for the element Iodine - I is provided by this page including scores of properties, element names in many languages, most known nuclides and .

The electron configuration of iodine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 5. Now in the next step, start drawing the orbital diagram for iodine. Draw orbital diagram. Before drawing the . Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.Iodine: Iodine is a p-block element having an atomic number 53 and an atomic symbol I. It belongs to the Halogen family i.e. Group-17. It is a non-metallic solid with a semi-lustrous appearance. Electronic configuration of Iodine: The electronic configuration of a neutral atom of Iodine is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 .electronic configuration for iodine Iodine (I) Oxygen, for example, has the electron configuration 1s 2 2s 2 2p 4, whereas the oxygen anion has the electron configuration of the noble gas neon (Ne), . Example \(\PageIndex{2}\): Determining the Electronic Structure of Anions. Selenium and iodine are two essential trace elements that form anions. Write the electron configurations of the .The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ). The electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements contains all the elements in increasing order of atomic number.. To save room, the configurations are in noble gas shorthand.This means part of the electron configuration has been replaced with the .
Shop the Color imageCLASS MF644Cdw from Canon U.S.A., Inc. This printer offers feature-rich capabilities with high-quality imaging and minimal maintenance. . Laser Beam Printing. PRINT SPEED 4: Up to 22 ppm (Letter); Up to 17.9 ppm (Legal) FIRST PRINT-OUT TIME: Approx. 10.3 Seconds. RECOMMENDED MONTHLY PAGE VOLUME:
electronic configuration for iodine|Iodine (I)